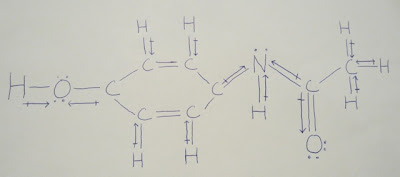
Lewis Structure of Acetaminophen
Nature of the bonds
Hydrogen to Oxygen is a moderately polar covalent bond. (The difference in electronegativity is 1.24)
Hydrogen to Carbon is a strongly polar covalent bond. (The difference in electronegativity is 0.35)
Hydrogen to Nitrogen is a moderately polar covalent bond. (The difference in electronegativity is 0.84)
Oxygen to Carbon is a moderately polar covalent bond. (The difference in electronegativity is 0.89)
Carbon to Nitrogen is a strongly polar covalent bond. (The difference in electronegativity is 0.49)
Carbon to Carbon is a purely covalent bond. (The difference in electronegativity is 0.00)
VSEPR
Acetaminophen is a linear molecule. This means that all of its bonds have a 180 degree angle.
Polarity
Acetaminophen is a polar molecule. It is polar because certain spots on the molecule are more electronegative that others. You can clearly see this through the arrows that are drawn from the less electronegative element to the more electronegative element.
No comments:
Post a Comment